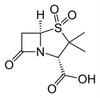
Sulbactam is an irreversible inhibitor of several bacterial penicillinases and cephalosporinases. In the presence of low concentrations of sulbactam, ampicillin and other β-lactams readily inhibit the growth of a variety of resistant bacteria that contain β-lactamases. Sulbactam was first developed by the central research division of Pfizer, Inc. and was first described in 1978.
Sulbactam used alone displays only weak antibacterial activity, with the notable exception of its potent effects on susceptible and resistant strains of Neisseria gonorrhoeae. Sulbactam sodium appears to be somewhat less potent but markedly more stable (in aqueous solution) than the β-lactamase inhibitor clavulanic acid.
Sulbactam is able to inhibit the most common forms of β-lactamase but is not able to interact with the AmpC cephalosporinase. Thus, it confers little protection against bacteria such as Pseudomonas aeruginosa, Citrobacter, Enterobacter, and Serratia, which often express this gene.
Sulbactam is commonly combined with ampicillin to form Ampicillin/Sulbactam (2:1). It does possess some antibacterial activity when administered alone, but it is too weak to have any clinical importance.
Recently, sulbactam has been used in treating Acinetobacter septicemia and is receiving renewed interest.
Synonyms: (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide, Betamaze, CP 45899, CP-45,899, Penicillanic acid 1,1-dioxide, Penicillanic acid S,S-dioxide, Penicillanic acid dioxide, Penicillanic acid sulfone
| Mechanism of Action | Sulbactam contains a β-lactam ring that binds strongly to β-lactamase at or near its activation site, thereby permanently inhibiting enzymatic activity. This action protects other β-lactam antibiotics (penicillins, cephalosporins, etc.) from β-lactamase catalysis, thereby enhancing their antibacterial activity. Sulbactam can also bind to and inhibit penicillin binding proteins PBP1 and PBP3. This antibacterial activity varies depending on the species. |
| Spectrum |
Antimicrobial activity Sulbactam inhibits a wide range of group 2 β-lactamases, including those from S. aureus, K. pneumoniae and B. fragilis. It is a good-to-moderate inhibitor of the TEM enzymes of groups 2b and 2be but has little effect on group 1, group 2br or group 3 β-lactamases. It does not induce the activity of cephalosporinases from Gram-negative bacteria but is a weak inducer of penicillinases from S. aureus. A concentration of 4–8 mg/L restores the activity of ampicillin for many β-lactamase-producing strains of S. aureus, H. influenzae, Mor. catarrhalis, enterobacteria and B. fragilis, but there is a large inoculum effect. |
| Microbiology Applications | Sulbactam is commonly used in combination with β-lactam antibiotics to prevent degredation by β-lactamase enzymes. |
| Plant Biology Applications | Sulbactam can be used in combination with ampicillin and cefoperazone to suppress β-lactamase activity when used in Agrobacterium-mediated plant genetic modifications (Ogawa and Mii, 2004). |
| Eukaryotic Cell Culture Applications | Studies have shown that sulbactam may protect cerebral neurons against oxygen-glucose deprivation (OGD) by up-regulating astrocytic GLT-1 expression via p38 MAPK signal pathway. |
| References |
Sulbactam Sodium. Chemical Book. N.p., 2010. Web. 23 Aug. 2012. Ogawa Y. and Mii M., Screening for highly active Ë-lactam antibiotics against Agrobacterium tumefaciens. Arch Microbiol, Vol. 181, pp. 331-336, 2004. |