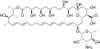
Nystatin, USP is a fungicidal and fungistatic polyene antifungal and growth promoter. It is composed of at least 85% Nystatin A1. It functions by accumulating cholesterol and thereby sequesters lipid from cell membranes. It can be used in quantum dot research, since cholesterol depletion could block several lipid raft-dependent endocytic pathways. It was discovered in 1950 by Rachel Fuller Brown and Elizabeth Lee Hazen and was the first polyene macrolide antifungal, named after New York State.
Nystatin, USP is soluble in ethanol and methanol.
We also offer:
| Mechanism of Action | Nystatin binds ergosterol in the fungal cell membrane forming pores and increasing permeability. The altered permeability is toxic to the fungi leading to growth inhibition or death. |
| Spectrum |
Nystatin primarily targets the cell membrane of Candida species. |
| Microbiology Applications | Nystatin is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against fungal isolates. Medical microbiologists use AST results to recommend treatment options. Representative effective ranges include:
For a representative list of Nystatin MIC values, click here. |
| Plant Biology Applications | Nystatin can be used in plant tissue culture to control fungal contamination. |
| Eukaryotic Cell Culture Applications | Nystatin is commonly used to prevent fungal contamination in cell culture.
Quantum dots can be used to deliver and monitor biomolecules, but the study of the uptake mechanism is just beginning. Researchers found that Nystatin is a highly selective inhibitor of the lipid raft-dependent pathway and the uptake of SR9 is a lipid raft-dependent process, thus it can be a valuable compound for quantum dot research (Xu et al, 2010). Nystatin can induce interleukin (IL)-1, IL-8, and tumor necrosis factor a secretion in TLR2-expressing THP1 cells. |
| Molecular Formula | C47H75NO17 (Nystatin A) |
| Solubility | Soluble in methanol and ethanol. Sparingly soluble in water. |
| Source | Streptomyces noursei and Streptomyces aureus |
| References |
Finkelstein A and Holz R (1973) Aqueous pores created in thin lipid membranes by the polyene antibiotics Nystatin and Amphotericin B. Mem. 2:377-408 PMID 4585230 Rice LB and Ghannoum MA (1999) Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 501-17 Sevtap Arikan (2002) In vitro activity of Nystatin compared with those of liposomal Nystatin, Amphotericin B, and Fluconazole against clinical Candida isolates. J. Clin. Microbiol. 40(4):1406-1412 Watts JW and King JM 1973) The use of antibiotics in the culture of non-sterile plant protoplasts. Planta. 113(30:271-277 Wilson ZA and Power JB (1989) Elimination of systemic contamination in explant and protoplast cultures of rubber (Hevea brasiliensis). Plant Ceil Rep. 7::622-662 |