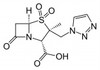
Tazobactam Sodium is the sodium salt of Tazobactam, a penicillanic acid sulfone derivative and ß-lactamase inhibitor with antibacterial activity. Tazobactam was discovered by Dr. R.G. Micetech at the University of Alberta in 1982. Tazobactam contains a ß-lactam ring and irreversibly binds to ß-lactamase at or near its active site. This protects other ß-lactam antibiotics from ß-lactamase catalysis. This compound is used in conjunction with ß-lactamase susceptible penicillins to treat infections caused by ß-lactamase producing organisms.
Tazobactam alone showed an MIC of ≤ 8 mg/liter (range 2 - 32 mg/liter) against several Acinetobacter baumannii strains. Tazobactam in combination with piperacillin, successfully restored the activity of piperacillin against β-lactamase-producing bacteria. Tazobactam exhibited inhibitory activity and protected piperacillin against Richmond and Sykes types II, III, IV and V β-lactamases, staphylococcal penicillinase and extended-spectrum β-lactamases. Tazobactam showed species-specific activity against class I chromosomally-mediated enzymes.
Tazobactam Sodium is soluble in water and methanol.