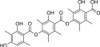
The fungal metabolite, thielavin A, and its relatives are glucose-6-phosphatase inhibitors. The three benzoic acid units are essential for inhibition. Thielavin A was originally isolated as a inhibitor of prostaglandin biosynthesis. The closely related thielavin B is a telomerase and cell wall transglycosylation inhibitor.
Thielavin A is soluble in ethanol, methanol, DMF and DMSO.
Thielavin A is soluble in ethanol, methanol, DMF and DMSO.