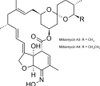
Milbemycin Oxime is a semi-synthetic, broad-spectrum macrocyclic lactone containing a 16-membered macrocyclic ring. Milbemycin Oxime is produced by fermentation of the soil-dwelling Streptomyces hygroscopicus subsp. aureolacrimosus followed by chemical modification (oxidation and oximation) of Milbemycin mixture A3 and A4 (~ 30:70 ratio). It is commonly used as an insecticide, parasiticide, and nematocide. Milbemycins are closely related to the avermectin class.
Milbemycin Oxime is sparingly soluble in DMSO.
We also offer:
- Milbemycin A3 (M057)
| Mechanism of Action | In invertebrates, macrocyclic lactones (MLs) can open ligand-gated chloride channels such as glutamate-sensitive chloride ion channels. When bound, MLs causes an influx of chloride into the parasite neurons leading to hyperpolarization, paralysis, and death.
In mammals, MLs bind to gamma-aminobutyric acid type A-gated chloride channels (GABAA receptors). GABA is the primary neurotransmitter in the brain. MLs are believed to bind to GABAA receptors, which are only present in the central nervous system. Milbemycin Oxime has surprising intrinsic antifungal activity. It inhibits fungal growth by inhibiting ATP-binding cassette (ABC) transporters. Fungicidal activity could be related to reactive oxygen species (ROS) species formation in these species. |
| Spectrum | Milbemycin Oxime is active against arthropods, nematodes, insects, and mites (Sarcoptes, Demodex). It is also active against fungi including C. albicans, C. glabrata. |
| Impurity Profile | Impurity G: ≤ 2.0% Impurity E: ≤ 0.7% Impurity H: ≤ 0.5% Impurity I: ≤ 0.5% Any other impurity: ≤ 0.2% Total impurities: ≤ 3.5% |
| Molecular Formula | Milbemycin A3 - C31H43NO7 Milbemycin A4 - C32H45NO7 |
| Solubility | Very soluble in anhydrous ethanol and ethyl acetate. Soluble in ethanol, methanol and DMF. Sparingly soluble in DMSO. |
| Assay | (A3 + A4): 95.0 - 102.0% A3 Ratio: ≤ 0.20 A4 Ratio: ≥ 0.80 |
| Residual Solvents |
Ethanol: ≤ 5000 ppm |
| References |
Humbert-Droz E, Buscher G, Cavalleri D, and Junquera P (2004) Efficacy of Milbemycin Oxime against fourthstage larvae and adults of Ancylostoma tubaeforme in experimentally infected cats. Vet. Rec. 154(5):140-143 Kornis GI (1995) Avermectins and Milbemycins. In: Godfrey CRA, ed. Agrochemicals from natural products. Marcel Dekker. New York, NY pp 215–255 Merola VM and Eubig PA (2012) Toxicology of avermectins and milbemycins (macrocylic lactones) and the role of P-glycoprotein in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 42(2): 313-333 Nakao T, Banba and Hirase K (2015) Comparison between the modes of action of novel meta-diamide and macrycyclic lactone insecticides on the RDL GABA receptor. Pest. Biochem. and Physiol. 120:101-108 PMID 25987227 Okazaki T et al.(1983) Milbemycins, a new family of macrolide antibiotics: Producing organism and its mutants J Antibiot. 36:438 PMID 6853372 Silva, LV et al (2013) Milbemycins: More than efflux inhibitors for fungal pathogens. Antimicrob. Agents Chemother. 57(2):873-886 PMID 23208712 Takiguchi Y et al (1980) Milbemycins, a new family of macrolide antibiotics: Fermentation, isolation and physico-chemical properties. J Antibiot. 33:1120 PMID 7451362 Takiguchi Y et al (1983) Milbemycins, a new family of macrolide antibiotics. Fermentation, isolation and physico-chemical properties of Milbemycins D, E, F, G, and H. J Antibiot. (Tokyo). 36(5):502-508 PMID 6874568 Tsukamoto Y. et al (1991) Synthesis of 5-keto-5-oxime derivatives of Milbemycins and their activities against microfilariae. Agric. Biol. Chem 55(10):2615-2621 PMID 1368759 Vercruysse J, Rew RS, eds. (2002) Macrocyclic lactones in antiparasitic therapy. New York: CABI |