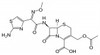
Cefotaxime Sodium, USP is broad-spectrum, third-generation cephalosporin. It interferes with bacterial peptidoglycan synthesis. Cefotaxime Sodium is freely soluble in aqueous solution.
Cefotaxime Sodium, USP conforms to United States Pharmacopoeia specifications.
We also offer:
- Cefotaxime ReadyMadeTM Solution (C293)
| Mechanism of Action | Like β-lactams, cephalosporins interfere with PBP (penicillin binding protein) activity involved in the final phase of peptidoglycan synthesis. PBP’s are enzymes which catalyze a pentaglycine crosslink between alanine and lysine residues providing additional strength to the cell wall. Without a pentaglycine crosslink, the integrity of the cell wall is severely compromised and ultimately leads to cell lysis and death. Resistance to cephalosporins is commonly due to cells containing plasmid encoded β-lactamases. Cefotaxime Sodium however, is largely resistant to β-lactamases. |
| Spectrum | Cefotaxime Sodium has broad-spectrum activity against a wide variety of Gram-positive and Gram-negative bacteria. However, unlike many cephalosporin antibiotics, Cefotaxime Sodium is not effective against Pseudomonas aeruginosa. |
| Microbiology Applications | Cefotaxime Sodium is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-positive and Gram-negative microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
|
| Plant Biology Applications | Cefotaxime is often used in Agrobacterium tumefaciens mediated transformations to combat bacterial growth. It is also sometimes used in combination with vancomycin as it has a synergistic effect. |
| Molecular Formula | C16H16N5NaO7S2 |
| Impurities | Chromatographic Purity: Single Impurity: ≤1.0% Total Impurities: ≤3.0% |
| References |
Georgopapadakou NH (1992) Mechanisms of action of cephalosporin 3'-quinolone esters, carbamates, and tertiary amines in Escherichia coli. Antimicrob. Agents. Chemother. 37(3): 559-65 PMID 8384817 Mathias RJ and Boyd LA (1986) Cefotaxime stimulates callus growth, embryogenesis and regeneration in hexaploid bread wheat (Triticum aestivum L. em Thell). Plant Sci. 46:217-223 Ferrer-González E, Kaul M, Parhi AK, LaVoie EJ and Pilch DS (2017) Lactam antibiotics with a high affinity for PBP2 act synergistically with the FtsZ-targeting agent TXA707 against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 61(9):e00863-17 PMID 28630190 |