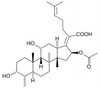
Fusidic Acid Sodium is a bacteriostatic steroid triterpenoid antibiotic of the fusidane class which inhibits protein synthesis in Gram-positive bacteria. It can be used to study the effects of compound combinations on bacterial growth, and gene regulation. Fusidic Acid suppresses nitric oxide toxicity in pancreatic islet cells. The product is freely soluble in water.
| Mechanism of Action | Fusidic Acid inhibits protein synthesis by preventing translocation by inhibiting ribosomal elongation factor G. |
| Spectrum | Fusidic Acid is a narrow-spectrum agent that that is bacteriostatic against Gram-positive bacteria but is primarily used against such as S. aureus and coagulate-negative species. It is also active against Gram-positive anaerobes but does not have activity against Gram-positive aerobes. |
| Impurity Profile | Impurity A| ent-(24SR,17Z)-16a-(acetoxy)-3b,11b,24,25-tetrahydroxy-4b,8,14-trimethyl-18-nor-5b,10a-cholest-17(20)-en-21-oic acid (24,25-dihydroxyfusidic acid)|||| Impurity C| ent-(17Z) -3b,11b-dihydroxy-17-[(6S)-6-(1-Hydroxy-1-methylethyl)-2-oxodihydro-2H-pyran-3(4H)-ylidene]-4b,8,14-trimethyl-18-nor-5b,10a-androstan-16a-yl acetate ((24R)-24,25-dihydroxyfusidic acid 21,24-lactone)|||| Impurity D| ent-(17Z) -3b,11b-dihydroxy-17-[(6R)-6-(1-hydroxy-1-methylethyl)-2-oxodihydro-2H-pyran-3(4H)-ylidene]-4b,8,14-trimethyl-18-nor-5b,10a-androstan-16a-yl acetate ((24S)-24,25-dihydroxyfusidic acid 21,24-lactone|||| Impurity E| ent-(17Z) -16a-(acetyloxy)-3b,11b,27-trihydroxy-4b,8,14-trimethyl-18-nor-5b,10a-cholesta-17(20),24-dien-21-oic acid (27-hydroxyfusidic acid)|||| Impurity F| ent-(17Z) -16a-(acetyloxy)-3b,11b,27-trihydroxy-4b,8,14-trimethyl-18-nor-5b,10a-cholesta-17(20),24-dien-21-oic acid (27-hydroxyfusidic acid)|||| Impurity K| ent-(17Z) -3b,11b,16a-trihydroxy-4b,8,14-trimethyl-18-nor-5b,10a-cholesta-17(20),24-dien-21,16-lactone(16-deacetylfusidic acid 21,16-lactone)|||| Impurity UN1| Fusidate Sodium|||| |
| Microbiology Applications | Fusidic Acid Sodium is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram-positive microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options. Representative MIC values include:
For a representative list of Fusidic Acid MIC values, click here.
Fusidic Acid resistance has emerged and given rise to methicillin-resistant Staphylocccus aureus (MRSA) and methicillin-susceptible S. aureus. Key resistance determinants include fusA gene mutations, encoding for elongation factor G, and plasmid-mediated resistance (ie. acquisition of resistance gene fusB).
Susceptibility testing in vitro is affected by the presence of blood or serum due to the high protein binding of Fusidic Acid. There is also a modest inoculum effect.
|
| Eukaryotic Cell Culture Applications | Fusidic Acid suppresses nitric oxide toxicity in pancreatic islet cells. It may protect islet beta cells from destruction in type 1 (insulin-dependent) diabetes mellitus. Fusidic Acid dose-dependently inhibited lysis of isolated islet cells by activated macrophages, a process mediated by nitric oxide. No protection was observed when cells were exposed to oxygen radicals or the alkylating beta cell toxin streptozotocin. The suppression of nitric oxide was not due to the compound's inhibition of protein biosynthessis thus it represents a yet unknown activity for this compound. |
| References |
Burkart V et al (1994) Fusidic acid suppresses nitric oxide toxicity in pancreatic islet cells. Biochem Pharmacol. 48(7):1379-1385 PMID 7945436 Collignon P and Turnidge J (1999) Fusidic Acid in vitro activity. Int. J. Antimicrob. Agents 12(2):45-58 Howden BP and Grayson ML (2006) Dumb and dumber--the potential waste of a useful antistaphylococcal agent: emerging Fusidic Acid resistance in Staphylococcus aureus. Clin Infect Dis. 42(3):394-400 PMID 16392088 |
| MIC | Bacillus subtilis| 50 - ?| 751| Borrelia burgdorferi S.L.| >4 - >4| 1129| Clostridium difficile| 0.0156 - 2| 492| Edwardsiella hoshinae| 32 - 64| 1409| Edwardsiella ictaluri| 0.13 - 64| 1409| Edwardsiella tarda| 32 - 64| 1409| Enterococcus faecalis (ATCC 29212)| 2 - ?| 401| Escherichia coli (C921-61)| 1024 - ?| 955| Escherichia coli (K-10)| >1024 - ?| 955| Escherichia coli (K-12)| 1024 - ?| 955| Escherichia coli (NCTC 10418)| >128 - ?| 401| Haemophilus influenzae| 2 - >16| 120| Moraxella catarrhalis| ≤0.03 - 0.25| 120| Salmonella typhimurium (LT2)| >1024 - ?| 955| Staphylococci| 0.03 - 128| 401| Staphylococcus aureus| 0.12 - >32| 604| Staphylococcus aureus| 0.13 - ?| 766| Staphylococcus aureus| 50 - ?| 751| Staphylococcus aureus (10389)| 0.12 - ?| 231| Staphylococcus aureus (10391)| >4 - ?| 231| Staphylococcus aureus (10397)| 0.12 - ?| 231| Staphylococcus aureus (10413)| 0.12 - ?| 231| Staphylococcus aureus (10467)| 0.12 - ?| 231| Staphylococcus aureus (10774)| 4 - ?| 231| Staphylococcus aureus (8249)| 0.06 - ?| 231| Staphylococcus aureus (8251)| 0.12 - ?| 231| Staphylococcus aureus (8258)| 0.06 - ?| 231| Staphylococcus aureus (ATCC 25923)| 0.12 - ?| 231| Staphylococcus aureus (ATCC 25923)| 0.12 - ?| 401| Staphylococcus aureus (ATCC 29213)| 0.06 - ?| 401| Staphylococcus aureus (ATCC 6571)| 0.06 - ?| 401| Staphylococcus aureus (community-acquired + methicillin-resistant)| 0.12 - 0.25| 604| Staphylococcus aureus (methicillin-resistant + mupirocin-resistant)| 0.12 - 2| 120| Staphylococcus aureus (methicillin-resistant)| 0.12 - >32| 604| Staphylococcus aureus (methicillin-resistant)| 0.12 - 2| 120| Staphylococcus aureus (methicillin-susceptible)| 0.12 - 16| 604| Staphylococcus aureus (methicillin-susceptible)| 0.12 - 1| 120| Staphylococcus aureus (vancomycin-intermediate-susceptible)| 0.062 - >64| 1060| Staphylococcus epidermidis| 0.12 - >16| 120| Staphylococcus epidermidis| 0.13 - ?| 766| Staphylococcus epidermidis (vancomycin-intermediate-susceptible)| 0.125 - 0.25| 1060| Staphylococcus haemolyticus (vancomycin-intermediate-susceptible)| 0.062 - 0.125| 1060| Staphylococcus pyogenes| 6.3 - ?| 766| Staphylococcus saprophyticus| 0.25 - 8| 120| Staphylococcus spp. (coagulase negative)| 0.06 - 32| 604| Streptococci (Viridans group)| 16 - >32| 120| Streptococcus agalactiae| 16 - 32| 120| Streptococcus pneumonia| 4 - 32| 120| |