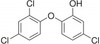
Irgasan (Triclosan) is a broad spectrum antibacterial agent that inhibits bacterial fatty acid synthesis. Irgasan is slightly soluble in water and freely soluble in ethanol and methanol.
| Mechanism of Action | Irgasan demonstrates a bacteriostatic effect by binding to the enoyl-acyl carrier protein reductase (enoyl-ACP), an enzyme involved in fatty acid synthesis. After forming a complex, nicotinamide adenine dinucleotide (NAD) binds and prevents enoyl-ACP from participating in the fatty acid synthesis cycle which inhibits bacterial cell growth. Triclosan permeabilizes the bacterial envelope. |
| Spectrum | Irgasan is active against Gram-negative bacteria, Gram-positive bacteria, and fungi |
| Microbiology Applications | Irgasan is used as a media supplement in Pseudomonas and Yersinia isolation media. During the 1990s, bacterial isolates with reduced susceptibility to Irgasan were produced by repeated exposure to sublethal concentrations. Since 2000, a number of studies have verified resistance among dermal, intestinal, and environmental microorganisms (Yazdankhah, 2006). Resistance in E. coli is acquired through a missense mutation in the fabl gene (Heath et al, 2010). |
| Eukaryotic Cell Culture Applications | A widely used model for proliferative epidermis in tissue engineering are the HaCa T keratinocytes. The influence of Irgasan on the cell growth, viability, and proliferation of these keratinocytes was reviewed, since the formation of biofilms pose a serious difficulty in tissue replacement. Collagen-cell-attachment properties on the atelocollagen matrix were not affected by this biocide. Thus, sample type could serve as ‘antimicrobial substrates’, and play a role in the design of novel antimicrobial biomaterials suitable for tissue engineering. (Lopez-Garcia J et al, 2014). |
| Molecular Formula | C12H7Cl3O2 |
| Solubility | Basic solution: Soluble Diethyl ether: Soluble Ethanol: Soluble Methanol: Soluble Water: Slightly soluble |
| Impurity Profile | Residue on Ignition: ≤0.1% Heavy Metals: ≤0.002% |
| References |
Heath RJ (2010) Mechanism of Triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274(16):11110-4. PMID 10196195
Yazdankhah SP (2006) Triclosan and antimicrobial resistance in bacteria: An overview. Microb Drug Resist. 12(2):83-90 PMID 16922622 |
| MIC | Candida albicans| 10 - 33| 693| Candida guilliermondii| 10 - 33| 693| Candida parapsilosis| 30 - ?| 693| Candida tropicalis| 10 - ?| 693| Escherichia coli| 0.004 - ?| 136| Escherichia coli (ATCC 25922)| 0.13 - ?| 186| Escherichia coli (N)| 0.03 - ?| 186| Escherichia coli (TEM-1)| 0.03 - ?| 186| Haemophilus influenzae| ≤0.06 - ?| 1281| Salmonella typhimurium (L133)| 0.12 - ?| 1008| Salmonella typhimurium (SL1344)| 0.12 - ?| 1008| Staphylococcus aureus| ≤0.06 - ?| 1281| Staphylococcus aureus (ATCC 11632 + methicillin-susceptible)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 13301 + methicillin-susceptible)| 2 - ?| 186| Staphylococcus aureus (ATCC 14154 + methicillin-susceptible)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 33591 + methicillin-resistant)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 33592 + methicillin-susceptible)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 33593 + methicillin-resistant)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 33594 + methicillin-susceptible)| 4 - ?| 186| Staphylococcus aureus (ATCC 43300 + methicillin-susceptible)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 700698 + methicillin-resistant)| 0.063 - ?| 186| Staphylococcus aureus (ATCC 700787 + methicillin-resistant)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 700788 + methicillin-resistant)| ≤0.016 - ?| 186| Staphylococcus aureus (ATCC 700789 + methicillin-resistant)| <0.016 - ?| 186| Staphylococcus aureus (JSB20013)| 0.125 - ?| 748| Staphylococcus aureus (RN4220/tet(A)/pYH4)| 0.125 - ?| 748| |