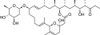
Venturicidin B (Aabomycin A2) is a 20-member macrolide glycoside compound that was originally isolated from a Streptomyces sp.by Glaxo Labs in 1961. Venturicidin B is a potent antifungal and toxic antibiotic compound, that has also shown cytotoxicity to trypanosomes.
Venturicidin B is a potent inhibitor of bacterial and mitochondrial ATP-synthase complexes acting on the F0 membrane sector, with experiments suggesting that the compound strongly inhibits ATP-driven proton transport and ATP hydrolysis. Venturicidin B demonstrates the ability to drastically decrease the open probability of voltage sensitive K+ channels. Venturicidin B is an inhibitor of ATP5 as well as a potential inhibitor of E. coli H+-ATPase.
Venturicidin B is soluble in ethanol, methanol, DMF and DMSO.
| Mechanism of Action | Venturicidin B binds to the subunit-c of the coupling factor o, or Fo of mitochondrial and bacterial ATP synthase complex. Once bound to the ATP synthase, Venturicidin B blocks proton translocation and inhibits ATP synthesis in both fungi and bacteria. |
| Spectrum | Venturicidin B is potent inhibitor of fungal and bacterial strains and has low toxicity for higher plants and animals. |
| Plant Biology Applications | Venturicidin protects plants from infection with pathogenic fungi such as Erysiphe graminis, Erysiphe cichoracearum, Podoshpaera leucotricha and Botrytis cinerea. It is also effective against fungi in the genus Venturia which can cause apple scab. |
| References | Studies on the mechanism of oxidative phosphorylation. ATP synthesis by submitochondrial particles inhibited at F0 by venturicidin and organotin compounds. Matsuno-Yagi A. & Hatefi Y. J. Biol. Chem. 1993, 268, 6168. Potassium selective and venturicidin sensitive conductances of Fo purified from bovine heart mitochondria, reconstituted in planar lipid bilayers. Miedema H. et al. Biochem. Biophys. Res. Commun. 1994, 203, 1005. Amino acid substitutions in mitochondrial ATP synthase subunit 9 of Saccharomyces cerevisiae leading to venturicidin or ossamycin resistance. Galanis M. et al. FEBS Lett. 1989, 249, 333. |