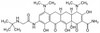
Tigecycline is a broad spectrum glycylcycline antibiotic useful for multi-drug resistant organisms and approved by the FDA in June 2005. Tigecycline is a semisynthetic derivative of tetracycline, that is structurally similar to minocycline; however, it contains a large glycylamido group at the D-9 position. This substitution is thought to be the reason behind its broad-spectrum activity.
Tigecycline is a protein synthesis inhibitor, that show bacteriostatic activity against both Gram-positive and Gram-negative bacteria. It was designed be less affected by the two major tetracycline-resistance mechanisms, ribosomal protection and efflux. Additionally, Tigecycline is not affected by resistance mechanisms such as beta-lactamases (including extended spectrum beta-lactamases), target-site modifications, macrolide efflux pumps or enzyme target changes (e.g. gyrase/topoisomerases). However, some ESBL-producing isolates may confer resistance to Tigecycline via other resistance mechanisms. Tigecycline resistance in some bacteria (e.g. Acinetobacter calcoaceticus-Acinetobacter baumannii complex) is associated with multi-drug resistant (MDR) efflux pumps.
Tigecycline has recently shown anti-tumor properties and is being evaluated for Tigecycline’s inhibitory effects on several activating signaling pathways and abnormal mitochondrial function in cancer cells.
Tigecycline is soluble in water (0.45 mg/mL) and DMSO (>3 mg/mL).
This product is considered a dangerous good. Quantities above 1 g may be subject to additional shipping fees. Please contact us for specific questions.
| Mechanism of Action | Tigecycline inhibits protein translation in bacteria by binding to the 30S ribosomal subunit and blocking entry of amino-acyl tRNA molecules into the A site of the ribosome. This prevents incorporation of amino acid residues into elongating peptide chains. In general, Tigecycline is considered bacteriostatic; however, Tigecycline has demonstrated bactericidal activity against isolates of S. pneumoniae and L. pneumophila. |
| Spectrum | Tigecycline has broad-spectrum activity against most Gram-positive and Gram-negative bacteria including multi-drug resistant organisms such as MRSA. Tigecycline has also been found to be effective against carbapenem resistant Enterobacteriaceae or CRE. CRE is a "superbug" which possesses NDM-1 or KPC genes which encode New Delhi Metallo-beta-lactamase or Klebsiella pneumoniae cabapenemase respectively; two enzymes which render nearly all beta-lactam antibiotics useless. |
| Microbiology Applications | Tigecycline is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against Gram positive and Gram negative microbial isolates. Tigecycline has also shown high potency against high-resistant superbug strains. Medical microbiologists use AST results to recommend antibiotic treatment options for infected patients. Representative MIC values include: Staphylococcus aureus (methicillin resistant) 0.03 µg/mL - 2 µg/mL Streptococcus pneumoniae 0.015 µg/mL – 2 µg/mL For a complete list of Tigecycline MIC values, click here. |
| Cancer Applications | An increasing number of studies have emphasized the anti-tumor effects of Tigecycline. The inhibitory effects of Tigecycline on cancer depend on activating several signaling pathways and abnormal mitochondrial function in cancer cells. Tigecycline has shown anti-tumor activity against different cancer types, including acute myeloid leukemia (AML), glioma, non-small cell lung cancer (NSCLC), among others. |
| Molecular Formula | C29H39N5O8 |
| References | Da Silva LM (2010) Tigecycline: A review of properties, applications, and analytical methods. Therapeutic Drug Monitoring 32.3 282-88 Greer, ND (2006) Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Proc.(Bayl.Univ.Med.Cent.) 19, 155-161 Milatovic, D., Schmitz, F.J., Verhoef, J., et al. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrobial Agents and Chemotherapy 47(1), 400-404 (2003). Theriot, C.M., Schumacher, C.A., Bassis, C.M., et al. Effects of tigecycline and vancomycin administration on established Clostridium difficile infection. Antimicrob. Agents Chemother. 59(3), 1596-1604 (2015). Stein, G.E., and Craig, W.A., 2006. Clin. Infect Dis. 43, 518. Pankey, George A. "Tigecycline." Journal of Antimicrobial Chemotherapy 56.3 (2005): 470-80. Web. 10 Apr. 2013. Livermore, D.M., 2005. J. Antimicrob. Chemother. 56, 611. |
| Impurities | 9-Amino-Minocycline: Not more than 0.15% Tigecycline epimers: Not more than 1.0% 9-nitro-Minocycline: Not more than 0.1% Any individual |
| MIC | Bacillus spp.| <0.015 - 0.12|| Bacteroides caccae| ≤0.06 - 8|| Bacteroides distasonis| ≤0.06 - 16|| Bacteroides eggerthii| 0.25 - 8|| Bacteroides fragilis| ≤0.06 - 32|| Bacteroides merdae| ≤0.06 - 8|| Bacteroides ovatus| ≤0.06 - 16|| Bacteroides stercoris| 0.25 - 8|| Bacteroides thetaiotaomicron| ≤0.06 - 32|| Bacteroides uniformis| ≤0.06 - 16|| Bacteroides vulgatus| ≤0.06 - 16|| Campylobacter coli| 0.03 - 0.06|| Campylobacter jejuni| 0.03 - 0.06|| Citrobacter freundii| 0.25 - 4|| Citrobacter koseri| 0.25 - 4|| Citrobacter spp. | 0.06 - 4|| Clostridium difficile| ≤0.06 - 2|| Clostridium innocuum| 0.125 - 1|| Clostridium perfringens| 0.25 - 4|| Corynebacterium jeikeium| <0.015 - 1|| Eikenella corrodens| ≤0.06 - 4|| Eleutherodactylus flavescens| 0.25 - 1|| Enterobacter aerogenes| 0.06 - 16|| Enterobacter agglomerans| 0.5 - 2|| Enterobacter amnigenus| 0.06 - 8|| Enterobacter asburiae| 0.06 - 8|| Enterobacter cancerogenus| 0.06 - 8|| Enterobacter cloacae| ≤0.008 - 16|| Enterobacter sakazakii| 0.06 - 8|| Enterobacter spp.| 0.06 - 8|| Enterobacter taylorae| 0.06 - 8|| Enterococcus avium| ≤0.016 - 2|| Enterococcus casseliflavus| <0.015 - 2|| Enterococcus durans| <0.015 - 2|| Enterococcus faecalis| ≤0.008 - 2|| Enterococcus faecium| <0.015 - 2|| Enterococcus gallinarum| ≤0.016 - 2|| Enterococcus hirae| ≤0.016 - 2|| Enterococcus mundtii| ≤0.016 - 2|| Enterococcus raffinosus| <0.015 - 2|| Escherichia coli| 0.015 - 4|| Eubacterium lentum| 0.125 - 0.5|| Finegoldia magna| 0.064 - 1|| Haemophilus influenzae| ≤0.008 - 4|| Hafnia alvei| 0.25 - 2|| JK diphtheroids| 0.12 - 4|| Klebsiella ornithinolytica| 0.12 - 4|| Klebsiella pneumonia| ≤0.008 - 16|| Klebsiella spp.| 0.06 - 8|| Lactobacillus spp.| 0.03 - 0.5|| Leuconostoc spp.| ≥0.12|| Listeria monocytogenes| 0.25 - 0.5|| Moraxella catarrhalis| ≤0.06 - 0.12|| Morganella morganii| 1 - 8|| Mycobacterium abscessus| ≤0.06 - 1|| Mycobacterium avium complex| ≥32|| Mycobacterium chelonae| <0.06 - ≤0.25|| Mycobacterium fortuitum| <0.06 - ≤0.25|| Mycobacterium kansasii| 8 - 32|| Mycobacterium lentiflavum| ≥32|| Mycobacterium marinum| 0.19 - 24|| Mycobacterium peregrinum| ≤0.06 - 0.12|| Neisseria meningitidis| 0.015 - 0.12|| Neisseria spp.| 0.12 - 0.5|| Pantoea agglomerans| 0.06 - 4|| Parvimonas micra| 0.016 - 0.38|| Pediococcus spp.| 0.03 - 1|| Peptoniphilus gorbachii | 0.016 - 0.094|| Peptoniphilus harei | 0.023 - 0.25|| Peptoniphilus ivorii | 0.032 - 0.25|| Peptoniphilus lacrimalis | 0.023 - 0.25|| Peptoniphilus octavius | 0.064|| Peptostreptococcus anaerobius| 0.064 - 0.125|| Peptostreptococcus spp.| 0.06 - 2|| Pneumococci | <0.016 - 0.125|| Propionibacterium acnes| 0.06 - 0.5|| Proteae| 0.06 - 16|| Proteus mirabilis| 0.25 - 16|| Proteus vulgaris| 2 - 8|| Providencia rettgeri| 2 - 8|| Providencia stuartii| 1 - 8|| Pseudomonas aeruginosa| ≤0.008 - >32|| Pseudomonas spp.| 0.5 - >8|| Ruminococcus gnavus| ≥0.094|| Salmonella spp.| 0.12 - 2|| Serratia fonticola| 0.25 - 8|| Serratia liquefaciens| 0.25 - 8|| Serratia marcescens| 0.12 - 8|| Serratia odorifera| 0.25 - 8|| Serratia odorifera| 0.25 - 8|| Serratia plymuthica| 0.25 - 8|| Serratia rubidaea| 0.25 - 8|| Shigella sonnei| 0.12 - 0.25|| Shigella spp.| 0.06 - 0.5|| Staphylococci| 0.03 - 8|| Staphylococcus aureus| <0.015 - 2|| Staphylococcus auricularis| 0.03 - 1|| Staphylococcus capitis| 0.03 - 1|| Staphylococcus chromogenes (coagulase-negative)| ≤0.06 - 0.5|| Staphylococcus cohnii| 0.03 - 1|| Staphylococcus epidermidis| ≤0.015 - 1|| Staphylococcus haemolyticus| 0.03 - 1|| Staphylococcus hominis| ≤0.06 - 1|| Staphylococcus lugdunensis| 0.03 - 1|| Staphylococcus saprophyticus| 0.03 - 1|| Staphylococcus sciuri| 0.03 - 1|| Staphylococcus simulans| 0.03 - 1|| Staphylococcus spp.| 0.03 - 1|| Staphylococcus warneri| 0.03 - 1|| Staphylococcus xylosus| 0.03 - 1|| Stenotrophomonas maltophilia| 0.12 - 8|| Streptococcus agalactiae| 0.015 - 1|| Streptococcus anginosus| <0.015 - 0.5|| Streptococcus bovis| 0.03 - 0.12|| Streptococcus constellatus| <0.015 - 0.5|| Streptococcus intermedius| <0.015 - 1|| Streptococcus mutans| <0.015 - 0.5|| Streptococcus oralis| <0.015 - 0.5|| Streptococcus parasanguis| <0.015 - 0.5|| Streptococcus pneumonia| ≤0.008 - 2|| Streptococcus pyogenes| 0.03 - 0.25|| Streptococcus uberis| <0.015 - 0.5|| Yersinia enterocolitica| 0.12 - 0.5|| |