About Oxazolidinones
Overview
The oxazolidinone class of antibiotics is a relatively recent addition to the antimicrobial world and have been found to be especially useful in treating infections caused by Gram-positive bacteria. The rather unique mode of action of oxazolidones gives them excellent activity against Gram-positive pathogens including Staphylococcus
aureus, Enterococcus spp, and Streptococcus
pneumoniae - all of which have shown alarming rates of antibacterial resistance in clinical settings. Oxazolidinones have also shown modest antibacterial activity against Gram-negative bacteria as well as mycobacteria.
Structure
Oxazolidinone compounds can be identified by the 2-oxazolidone in the structure. The class of antibiotics generally contains the 2-oxazolidone with a 4-substituted phenyl ring in the 3 position: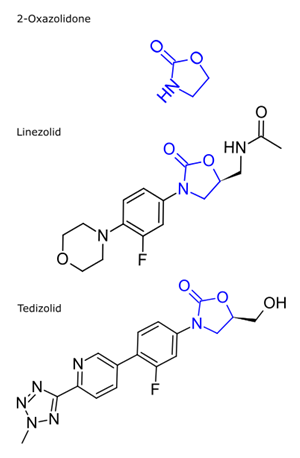
History
Antibacterial oxazolidinone compounds were first reported in the 1980's by DuPont and referred to as DuP 105 and DuP 721. Issues with toxicity in human clinical trials caused them to fall out of favor. The class was revisited years later and examined for higher efficacy and safety, leading to the discovery of two promising candidates, eperezolid and linezolid. Their initial tests were nearly identical in bioactivity and toxicity, so both were put into clinical testing. Linezolid had a minor advantage that allowed it to advance ahead of eperezolid: eperezolid would need to be administered three times a day and linezolid would only need to be administered twice a day. Linezolid became the first oxazolidinone approved by the FDA. [1]
In vitro activity and spectrum
The auspicious reputation of oxazolidinones is based off their tested and effective activity against multi-drug resistant Gram-positive bacteria. Highly effective activity against methicillin-resistant Staphylococcus
aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), vancomycin-intermediate S.
aureus (VISA), vancomycin-resistant S. aureus (VRSA) and vancomycin-resistant Enterococcus (VRE) has been reported. Oxazolidinones have excellent activity against Mycobacterium
tuberculosis (TB) with the MIC90 of linezolid in clinical isolates of M.
tuberculosis being as low as 0.5 µg/ml. Susceptibility to oxazolidinones extends to other rapid-growth mycobacteria, including M.
fortuitum third biovariant complex, M.
mucogenicum and M. Smegmatis group.
Other bioactivity of interest includes activity against Gram-negative pathogens Clostridium
difficile (C. diff), Bordetella pertussis (Pertussis or whooping cough) and Legionella spp, the causative of Legionnaire’s disease. [2],[3].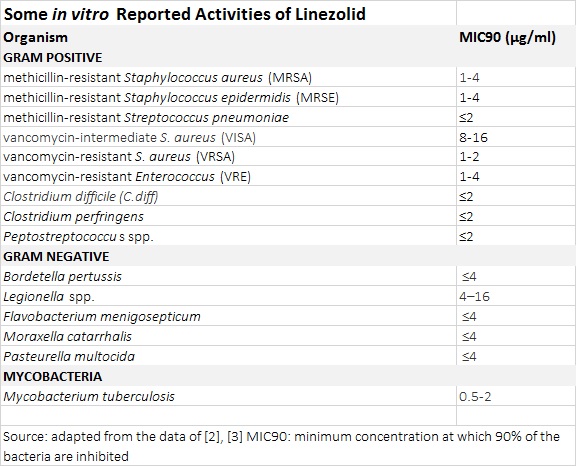
Bactericidal activity
Generally, oxazolidinones have been found to be bacteriostatic, exhibiting activity that stops bacterial replication without directly resulting in bacterial death (bactericidal). However, in some cases oxazolidinones have been found to be bactericidal depending on the species of bacteria. Linezolid is generally bacteriostatic against staphylococci and enterococci but bactericidal against streptococci such as S.
pneumoniae. [4]
Mechanism of action
The oxazolidinones are a class of bacterial protein synthesis inhibitors, but their uniqueness lies in how early in the process they start inhibiting synthesis. While tetracyclines block aminoacyl tRNA entry and macrolides cause premature termination, oxazolidinones prevent the assembly of the ribosome. Linezolid and eperezolid inhibit the initiation of ribosomal protein synthesis by binding to the 50S subunit, blocking the creation of the 70S initiation complex. This unique mechanism of action decreases the possibility that target bacteria will develop cross-resistance to other, similar antimicrobials. [3]
Current Oxazolidinones
Linezolid
Linezolid has seen incredible success. Pharmaceutical sales of brand-name Zyvox have been over $1 billion per year multiple years in a row. [1] Its broad antibiotic spectrum which is able to target multi-drug resistant pathogens is the basis of its popularity, especially with nosocomial antimicrobial resistances on the rise. Furthermore, it was the only FDA approved oxazolidinone on the pharmaceutical market for 14 years. It has an excellent adsorption rate, with mean bioavailability of 100%. [2] It has very little toxicity and few adverse effects in short-term treatment.
Tedizolid
Tedizolid is the second oxazolidinone to come on the market, with even more potency against staphylococci, streptococci and enterococci than its predecessor Linezolid. It retains more potency than linezolid against resistant strains, and selection of drug-resistant mutations was lower. The medical world is very relieved to have another oxazolidinone to combat multidrug resistant bacterial infections as new antimicrobials are always welcome in the arms-race against pathogenic resistance. [5]
Future oxazolidinone antibiotics
Considerations Going Forward
While many potential new members of the antibacterial oxazolidinone drug family have been considered over the last two decades, many were terminated because either the activity was not within the desired therapeutic range, they had low solubility or they were not significantly different than the currently available linezolid. Others simply did not meet the proper safety standards. [1] Linezolid’s use has been restricted from longer therapies like the treatment of Tuberculosis (TB) due to toxicity which limits its use to only a few weeks. Prolonged used of linezolid can cause myelotoxicity, cytopenia, neuropathies, lactic acidosis, and rhabdomyolysis. A focus on screening oxazolidinones for increased safety is a big part of research into new additions to this antibiotic family. [6]
MRX-I
MRX-I is a novel oxazolidinone developed by MicuRx Pharmaceuticals Inc. specifically with the attenuation of myelotoxicity and monoamine oxidase inhibition in mind. It has seen success in clinical trials with no adverse events. [7] MRX-I has seen excellent antibacterial and anti-mycobacterial activity, even outpacing Linezolid in its activity against MRSA. [8]
T145
T145 is a novel oxazolidinone developed on the heels of linezolid. It matches linezolid’s potency in vitro, but minimizes selection of drug-resistant mutants. The fewer drug-resistant mutants, the longer a drug will remain effective and useful for the healthcare world. [9]
AZD5847
AZD5847 improves on the in vitro anti-tuberculosis activity of linezolid, both intracellular and extracellularly. This increased potency could shorten treatment times. Furthermore, it has additive effects with current TB treatments, setting it up for combination therapy commonly used in the treatment of TB. [10]
TOKU-E News will continue to post new research into novel oxazolidinones and other antibiotics.
Sources:
[1] Shaw, K. J., & Barbachyn, M. R. (2011). The oxazolidinones: Past, present, and future. Annals of the New York Academy of Sciences, 1241(1), 48-70 doi.org/10.1111/j.1749-6632.2011.06330.x
[2] Tsuji, B. T., Pharm D, Kaatz, G. W., MD, & Rybak, M. J., Pharm D. (2014). Linezolid and Other Oxazolidinones. Retrieved from http://www.antimicrobe.org/d13.asp
[3] Diekema, D. J., & Jones, R. N. (2001). Oxazolidinone antibiotics. The Lancet, 358(9297), 1975-1982. doi.org/10.1016/s0140-6736(01)06964-1
[4] Pankey, G. A., & Sabath, L. D. (2004). Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram‐Positive Bacterial Infections. Clinical Infectious Diseases, 38(6), 864-870. doi.org/10.1086/381972
[5] Locke, J. B., Zurenko, G. E., Shaw, K. J., & Bartizal, K. (2013). Tedizolid for the Management of Human Infections: In Vitro Characteristics. Clinical Infectious Diseases, 58(Suppl 1). doi:10.1093/cid/cit616 [6] Hoagland, D. T., Liu, J., Lee, R. B., & Lee, R. E. (2016). New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Advanced Drug Delivery Reviews, 102, 55-72. doi.org/10.1016/j.addr.2016.04.026
[7] MicuRX Reports Positive Top-Line Results In Phase 2 Clinical Trial For Novel Antibiotic MRX-I In Complicated Skin And Soft Tissue Infections. (2015, August 31). Retrieved from http://www.prnewswire.com/ [Article written by MicuRx Pharmaceuticals, Inc.]
[8] Gordeev, M. F., & Yuan, Z. Y. (2014). New Potent Antibacterial Oxazolidinone (MRX-I) with an Improved Class Safety Profile. Journal of Medicinal Chemistry, 57(11), 4487-4497. doi.org/10.1021/jm401931e
[9] Kaushik, A., Heuer, A. M., Bell, D. T., Culhane, J. C., Ebner, D. C., Parrish, N., . . . Lamichhane, G. (2016). An evolved oxazolidinone with selective potency against Mycobacterium tuberculosis and gram positive bacteria. Bioorganic & Medicinal Chemistry Letters, 26(15), 3572-3576. doi.org/10.1016/j.bmcl.2016.06.019
[10] Balasubramanian, V., Solapure, S., Iyer, H., Ghosh, A., Sharma, S., Kaur, P., … Sambandamurthy, V. K. (2014). Bactericidal Activity and Mechanism of Action of AZD5847, a Novel Oxazolidinone for Treatment of Tuberculosis. Antimicrobial Agents and Chemotherapy, 58(1), 495–502. doi.org/10.1128/AAC.01903-13
