Gentamicin X2’s bioactivity has not been thoroughly researched, but it is well known as the precursor component to the very bioactive gentamicin C complex. The graphic below was obtained from a paper by Gu et al. and illustrates the biosynthetic scheme of the gentamicin C complex from Gentamicin X2 [7]: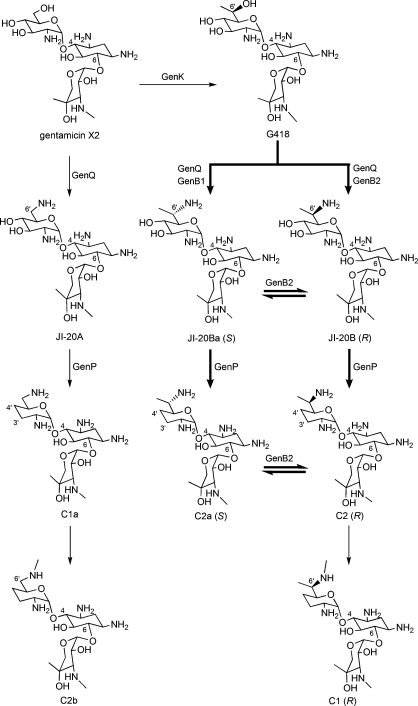
Studying the individual congeners of gentamicin allows us to fully explore the potential of gentamicin and is a promising way to increase its safety and therapeutic window. Similar research is being done with other antibiotic congeners and we hope to see exciting data in the future!
Resources:
[1] Vydrin, A. F., Shikhaleev, I. V., Makhortov, V. L., Shcherenko, N. N., & Kolchanova, N. V. (2003). Component Composition of Gentamicin Sulfate Preparations. Pharmaceutical Chemistry Journal, 37(8), 448-450. doi.org/10.1023/A:1027372416983
[2] Weinstein, M. J., Wagman, G. H., Oden, E. M., & Marquez, J. A. (1967). Biological activity of the antibiotic components of the gentamicin complex. Journal of Bacteriology, 94(3), 789–790.
[3] Sandoval, R. M. (2006). A Non-Nephrotoxic Gentamicin Congener That Retains Antimicrobial Efficacy. Journal of the American Society of Nephrology, 17(10), 2697-2705. doi.org/10.1681/asn.2005101124
[4] Isoherranen, N., Lavy, E., & Soback, S. (2000). Pharmacokinetics of Gentamicin C1, C1a, and C2 in Beagles after a Single Intravenous Dose. Antimicrobial Agents and Chemotherapy, 44(6), 1443-1447. doi.org/10.1128/aac.44.6.1443-1447.2000
[5] Steinman, A., Isoherranen, N., Ashoach, O., & Soback, S. (2010). Pharmacokinetics of gentamicin C1, C1a and C2 in horses after single intravenous dose. Equine Veterinary Journal, 34(6), 615-618. doi.org/10.2746/042516402776180160
[6] Fox, K. E., Brummett, R. E., & Brown, R. (1980). A Comparative Study of the Ototoxicity of Gentamicin and Gentamicin C1. Archives of Otolaryngology - Head and Neck Surgery, 106(1), 44-49. doi.org/10.1001/archotol.1980.00790250046009
[7] Gu, Y., Ni, X., Ren, J., Gao, H., Wang, D., & Xia, H. (2015). Biosynthesis of Epimers C2 and C2a in the Gentamicin C Complex. ChemBioChem, 16(13), 1933-1942. doi.org/10.1002/cbic.201500258
