Polymyxins have become their own family of lipopeptide antibiotics. While polymyxins A, B, C, D and E have been described, only B and E have been used clinically because they display the least nephrotoxicity. [1] Polymyxin E is sometimes referred to as colistin. Polymyxins B and E are made up of multiple congeners, each varying slightly in their amino acid component or fatty acid chain. [2] Here, you can see the structural relations between the polymyxin B components characterized by Orwa et al.: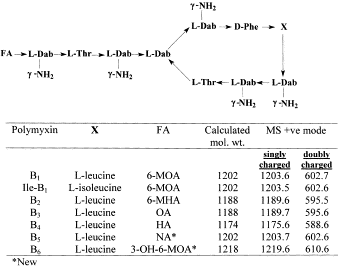
While Polymyxin B contains phenylalanine in position six, colistins contain D-leucine. [3] Polymyxins are highly surface-active, and have a detergent-like mechanism of action. They lyse the bacterial membrane, and are therefore considered bactericidal. Their antibacterial activity is antagonized by soap. [1]
The five main congeners of polymyxin B were tested for relative potency by Tam et al. against wild-type and drug-resistant Gram-negative bacteria. [4] It should be noted that polymyxin B has no activity against Gram-positive bacteria, so all gram-negative pathogens were tested [5]. Polymyxin B1, polymyxin B1-I, polymyxin B2, polymyxin B3 and polymyxin B4 demonstrated no notable difference in the in vitro potency among the bacteria tested.
Interestingly, Roberts et al found that the purified congeners performed slightly better than the clinical complexes of polymyxin B and polymyxin E. Their research suggests that the minor components in the commercial complexes, which can represent up to %30 of the lipopeptide content, don’t have the same bioactivity as the major components. [6]
Knowledge of the pharmacokinetics and pharmacodynamics of the polymyxins is limited due to lack of use in the last 50 years. Nephrotoxicity and neurotoxicity are the most common potential toxicities in treatment with the polymyxin complexes; neurotoxicity is much less common than nephrotoxicity and is usually mild, resolving after discontinuation of therapy. [5]
Researchers hope that they can attenuate toxicity in the polymyxin complexes by finding and using only the least toxic congeners for treatment. Pharmacokinetics studies comparing the individual components of the polymyxins can help determine the mechanism of toxicity. In an animal model, Abdelraouf et al found that the polymyxin B components polymyxin B1, polymyxin B1-I, and polymyxin B2/B3 appear to have similar pharmacokinetics. They stated that polymyxin B appears to preferentially accumulate in the renal tissue, which may be related to its nephrotoxicity. They also discovered that a mechanism other than renal excretion is likely involved in the elimination of polymyxin B from the body. [6] Roberts et also found polymyxin B1, polymyxin B2, polymyxin E1 and polymyxin E2 to have comparable nephrotoxicities in mice. [7]
The use of polymyxins is increasing with the increase of nosocomial multidrug-resistant infections, but our knowledge of their toxicity has fallen decades behind. [5] Study of the individual congeners of polymyxin can help us use our antibiotics in the smartest way possible to reduce toxicity. They can also be a great start for newly-derived, novel antibiotics. As their clinical use increases, we hope to see research surrounding the polymyxins increase as well.
Resources:
[1] Newton, B. A. (1956). The Properties and Mode of Action of the Polymyxins. Bacteriological Reviews, 20(1), 14–27.
[2] Orwa, J. A., Govaerts, C., Busson, R., Roets, E., Van Schepdael, A., & Hoogmartens, J. (2001). Isolation and structural characterization of polymyxin B components. Journal of Chromatography, 912(2), 369–373. http://doi.org/10.1016/s0021-9673(01)00585-4
[3] Falagas, M. E., & Vardakas, K. Z. (2014). Polymyxins - infectious disease and Antimicrobial agents. Retrieved from Infectious Disease & Antimicrobial Agents, http://www.antimicrobe.org/d05.asp
[4] Tam, V. H., Cao, H., Ledesma, K. R., & Hu, M. (2011). In Vitro potency of various Polymyxin B components. Antimicrobial Agents and Chemotherapy, 55(9), 4490–4491. http://doi.org/10.1128/aac.00119-11
[5] Zavascki, A. P., Goldani, L. Z., Li, J., & Nation, R. L. (2007). Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. Journal of Antimicrobial Chemotherapy, 60(6), 1206–1215. http://doi.org/10.1093/jac/dkm357
[6] Abdelraouf, K., He, J., Ledesma, K. R., Hu, M., & Tam, V. H. (2012). Pharmacokinetics and renal disposition of Polymyxin B in an animal model. Antimicrobial Agents and Chemotherapy, 56(11), 5724–5727. http://doi.org/10.1128/aac.01333-12
[7] Roberts, K. D., Azad, M. A. K., Wang, J., Horne, A. S., Thompson, P. E., Nation, R. L., … Li, J. (2015). Antimicrobial activity and toxicity of the Major Lipopeptide components of Polymyxin B and Colistin: Last-line antibiotics against Multidrug-Resistant gram-negative bacteria. ACS Infectious Diseases, 1(11), 568–575. http://doi.org/10.1021/acsinfecdis.5b00085
